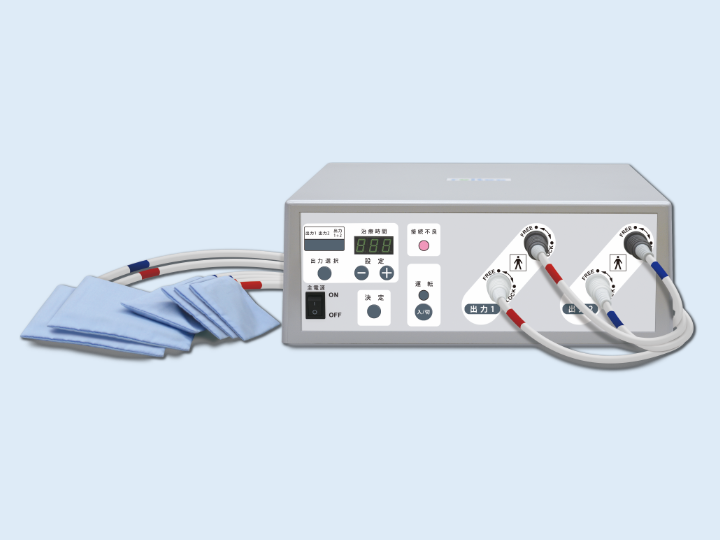
What is an RE therapeutic device?
“RE therapeutic device (Reducing Electron therapeutic device)” is RELTEC’s internal term for all RELTEC products that have a “transcutaneous electron supply function” for antioxidant purposes. RE therapy (Reducing Electron therapy)” means “transcutaneous electron therapy” proposed by Japanese M.D. Hiroshi Horiguchi1.
The RE therapeutic device has a transcutaneous electron supply function using microcurrent below 6 μA and negative high voltage and has continued to be improved over many years.
“Oxidized” means that electrons are removed, and “reduced” means that electrons are given. The RE therapeutic device’s transcutaneous electron supply function was developed to safely and effectively supply electrons to the human body.
Two types of RE therapeutic devices
RE therapeutic device certified as a medical device for professionals in Japan
Products that fall under this category have the following two functions and are approved as medical devices in Japan based on their negative voltage application function. Indications in Japan are insomnia, headache, stiff shoulders, and chronic constipation. Still, it is often used as an option of complementary and alternative medicine for home treatment by people who are dissatisfied with the standard of care.
- transcutaneous electron supply function
- Negative voltage application function
The negative potential application function is achieved not by the structure or parts of the main body of the therapeutic device, but by insulating the human body from the earth with an insulating mat as an accessory.
Electron supply device (non-medical device)
Products in this category have a transcutaneous electron supply function for antioxidants but do not have a negative voltage application function. Therefore, they are not medical devices in Japan.
The sameness and differences between the two types of RE therapeutic devices
Sameness― product structure and parts, etc.
- Product structure and parts
-
The structure and parts of the product bodies of the MD21 and HD21, or MsD and HsD, are the same. Therefore, regarding the transcutaneous electron supply function, which RELTEC believes is the original function of RE therapeutic devices, there is almost no difference between MD21 and HD21 and between MsD and HsD.
The only difference between the parts for medical devices and non-medical devices is that the former includes an insulating mat as an accessory. Using the insulating mat creates a negative voltage application function for medical devices. However, RELTEC is confident that the negative voltage application function does not affect the antioxidant effect of the RE therapeutic device.
- Manufacturing and quality control
-
All RE therapeutic devices are manufactured and quality-controlled at the RELTEC factory in Japan by national medical device manufacturing standards.
Differences – end user price, etc.
- Electron supply devices with low end-user price
-
RELTEC has set low-end user prices for the electron supply devices to provide the benefits of the RE therapeutic devices to as many people as possible. In particular, RELTEC has positioned the HsD, which is small, lightweight, highly portable, and easy to use, as a model for popularizing the RE therapeutic device and has set the price significantly lower than other models.
Product RE therapeutic device Medical device Electron supply device MD21
DetailsMsD
DetailsHD21
DetailsHsD
DetailsRecommended retail price excluding tax in Japan 1.1 million JPY 0.55 million JPY 0.9 million JPY 0.35 million JPY - Electron supply device for non-medical devices with no negative voltage application function
-
As mentioned above, the accessories for the electron supply device do not include an insulating mat to realize the negative voltage application function. Therefore, the electron supply device is not a medical device in Japan.
- Electron supply device with intentional partial performance degradation
-
Although the main structure and parts of the electron supply device and medical device are the same, the electron supply device is set to have partially lower performance than the medical device, as shown below. RELTEC has set the performance partially lower to the extent that the transcutaneous electron supply function is not substantially Impaired to increase the failure resistance of electron supply devices, which are used in more diverse environments than medical devices.
- The electron supply device has a slightly lower output voltage than the medical device.
- The electron supply device has a shorter maximum settable therapeutic time than the medical device.
Product RE therapeutic device Medical device Electron supply device MD21
DetailsMsD
DetailsHD21
DetailsHsD
DetailsNumber of output channels 2 1 2 1 Output voltage DC – 5.5 ± 0.5 kV DC – 5.0 ± 1.0 kV DC – 5.0 ± 1.0 kV DC – 4.5 ± 0.9 kV Maximum settable
therapeutic time99 min. 120 min. 60 min. 60 min. - HD21, where it is impossible to select a conductive plate with a higher output current value.
-
The conductive plate is an accessory that comes into direct contact with the human body to supply the current output from the RE therapeutic device’s main body to the human body. By changing the resistance value of this conductive plate, the current value supplied to the human body can be changed.
For the medical device MD21, you can choose between two types of condactive plates: type S (standard supply current value) and type H (higher supply current value than type S). On the other hand, for the electron supply device HD21, you can only choose type S. In addition, the conductive plates of the medical device MsD and the electron supply device HsD are both type H.
Which RE therapeutic device should you choose?
RELTEC recommends selecting the RE therapeutic device model based on your purpose of use and living conditions as follows.
| Model selection of RE therapeutic device | Purpose of use | ||
| Therapy of diseases | Health maintenance/promotion | ||
| It can be used in the supine position for a certain amount of time. | MD21 Details | HD21 Details | |
| It cannot be used in the supine position for a certain amount of time. | MsD Details | HsD Details | |
- Director of Horiguchi Clinic, Kenshokai Medical Corporation―3329-14, Kawatsu-cho, Sakaide-shi, Kagawa 762-0025 Japan ↩︎